| |
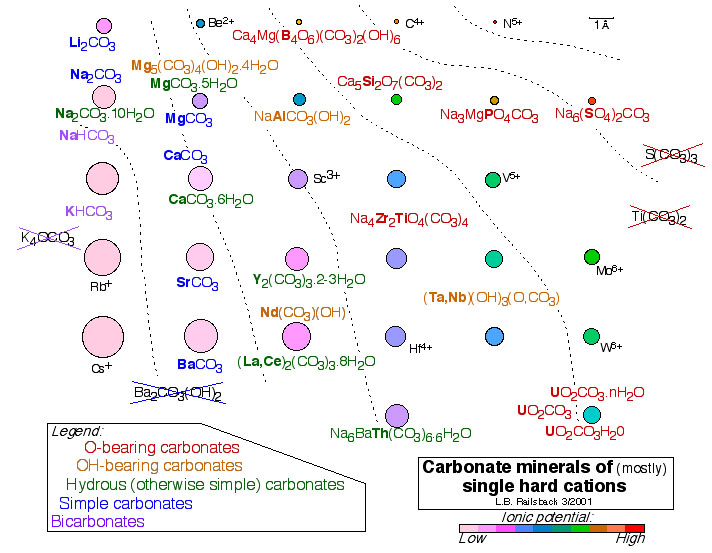
If you haven't seen enough mineralogy yet, this figure shows the chemistry of carbonate minerals, preferably of one cation but of two or even three as needed. It illustrates that minerals built of radicals of a cation of high ionic potential (here, of carbonate groups with C4+), have predictable chemistries. We're most familiar with the simple carbonates of divalent cations, but simple carbonates can also have Li+ or Na + (blue). Cations of lower ionic potential (e.g., K+) have H+ as well, presumably because the low density of positive charge provided by K+ doesn't shield the negative charge of O2-s sufficiently (purple). On the other hand, incorporating ions of higher ionic potential requires incorporation of water molecules (greens), OH-s (golds), and even extra O2-s to shield the positive charge of those cations of high ionic potential from the positive charge of C4+. Some crossed-out non-minerals illustrate the opposite - for example, that no simple carbonate can accept a cation of high ionic potential. All of this falls into one consistent pattern when the periodic table is arranged in terms of ions, as in the
Earth Scientist's Periodic Table of the Elements and Their Ions.
|