| |
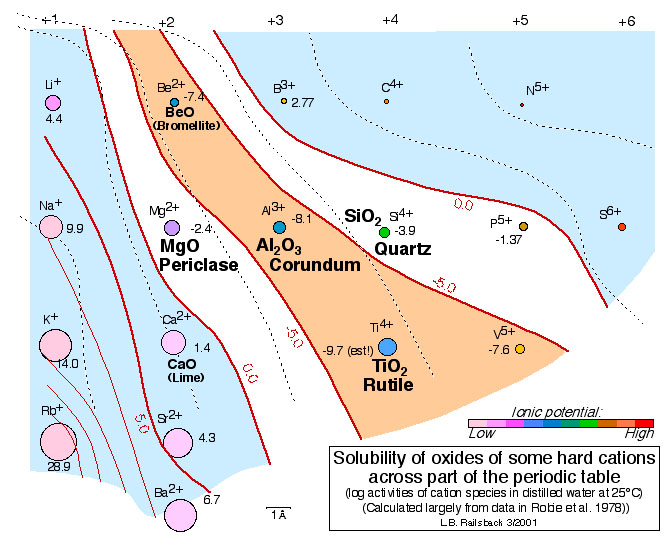
This figure shows variation in the solubility of oxides of hard cations. Mineral names are shown for minerals; many of the substances for which solubilities are shown aren't minerals (they don't occur in nature). The ions that make insoluble oxides are also the ions that are concentrated in soils and that enter early-forming phases in igneous rocks (as shown with brown and red symbols, respectively, on the
Earth Scientist's Periodic Table of the Elements and Their Ions). Note that the arrangement of ions in the Earth Scientist's Periodic Table of the Elements and Their Ions makes these solubilities follow a pattern easily predicted from the contours of equal ionic potential. It's much the same pattern as that in the melting temperatures of oxides shown in Accompanying Figure 7.
|