| |
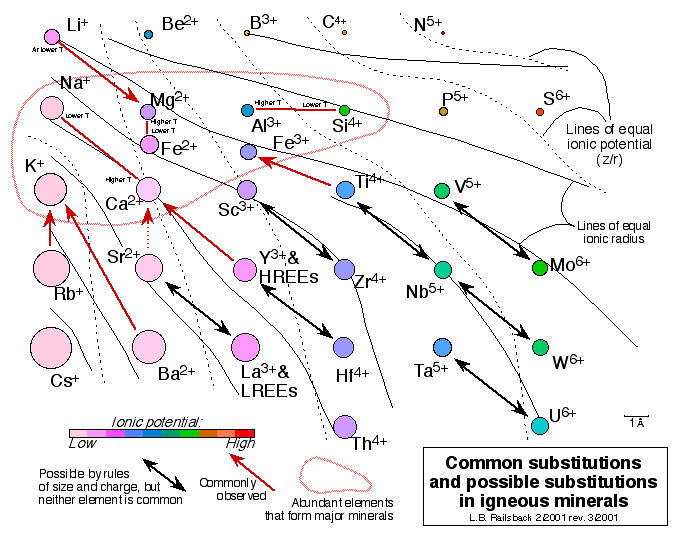
This figure shows patterns of substitution of ions in igneous minerals across an important part of the periodic table. Ions with a difference in charge of no more than 1 and with about the same size can substitute for each other. Some ions are so abundant that the have to make ignoeous minerals (as in the red circle). Others are outside the circle but can substitute for ones inside, and so they are "compatible" in early-forming igneous minerals (Y3+ and Ti4+ are good examples). Others have no such possibility of substitution and so are incompatible. Zr4+ is a great example, in that it can substitute for Sc3+ - but Sc3+ is so rare that substituting for it is meaningless. As a result, Zr4+ commonly remains in magmas until forming zircon very late in crystallization. These concepts lead on to Accompanying Figure 9 accompanying the
Earth Scientist's Periodic Table of the Elements and Their Ions.
|