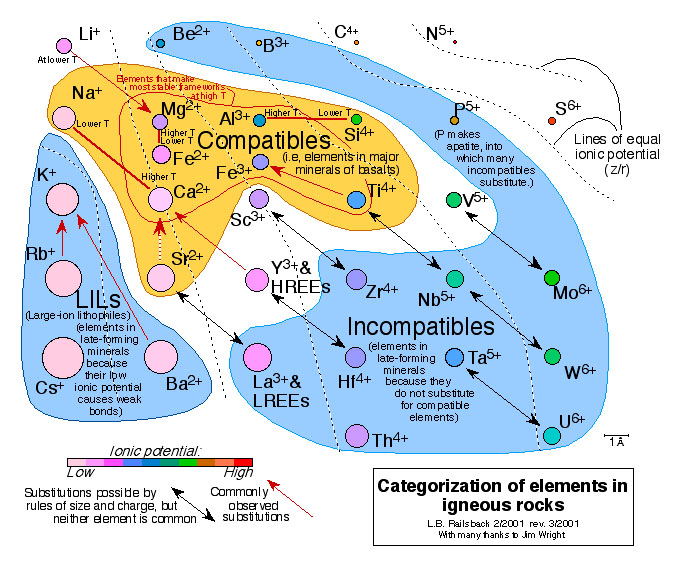
Note that some ions are more of less compatible than others. Na+, for example, enters plagioclase, but only at lower temperatures than Ca2+, because Na+ is farther from the "fairway" of intermediate ionic potential (roughly z/r = 5). On the other side of this figure, Si4+ likewise becomes more abundant than Al3+ in plagioclase at lower temperatures. That's consistent with the general greater abundance of Si4+ in igneous phases at lower temperatures, as seen in Accompanying Figure 10. If a compatible ion is one that enters early-forming igneous phases, then Si4+ is at best a borderline igneous phase. That seems like a paradox - Si4+ is an incompatible ion in crystallization of silicate magmas? - until one sees the borderline position of Si4+ on this figure and in general on the Earth Scientist's Periodic Table of the Elements and Their Ions.