|
This is the first in a pair of virtual field trips taking us to localities in the western United States at which travertine is forming today. This trip takes us along Fall Creek in southeastern Idaho. The second trip will be in northwestern Wyoming, at Mammoth Hot Springs in Yellowstone National Park.
|

|
|
So what's travertine? Definitions vary, but a relatively restrictive modern definition might be "a body of calcium carbonate precipitated from waters of a hot spring, and thus relatively solid and not porous". In contrast, tufa might be defined as ""a body of calcium carbonate precipitated from relatively cool earth-surface waters, and thus relatively porous with molds of plants and other macroscopic organisms". These definitions will come back to haunt us a little further along. Our travertine locality for this trip is east of Idaho Falls, Idaho, and up the valley of Fall Creek, a tributary of the Snake River at about 43° 23' N, 111° 30' W. Fall Creek runs at an elevation at about 6000 feet and cuts across ridges of the Caribou Range of the greater Rockies. Here's how things appear as we look up the valley of Fall Creek:
|
|
|

|
|
|
|
Going up Fall Creek, we come to the main attraction: a large body of travertine seen in the image below on the far side of the creek:
|
|
|

|
|
|
|
If we go downstream a little, we find that the creek is lined with travertine like the overhanging ledge in the image below. Note the stream of water emerging from the travertine in the middle of the picture - there's certainly a spring here!
|
|
|

|
|
|
|
Just a little farther downstream, the image below shows the layered nature of the travertine - it seems to have built upwards as much or more than outwards. Notice also the placid water of the stream above the rocks and the white look of the stream bottom near us, downstream from where the water tumbles over those rocks.
|
|
|

|
|
|
|
In our last view of the stream in the image below, we're again looking at the white bottom of the stream, downstream from where the water has tumbled over rocks. What causes the white color?
|
|
|

|
|
|
|
The white color on the stream's bottom is calcium carbonate coating the vegetation and rocks on the stream bottom. As the stream flows over a small falls, like that at the rocks in the images above, the water is agitated. That agitation releases CO2 from the water, just as a shaken soft drink or beer will fizz with the CO2 exsolved. Releasing CO2 from the water drives the following chemical reaction to the right:
This is the fundamental equation for precipitation of calcium carbonate (CaCO3). Here it explains why calcium carbonate coats the stream bottom. A little more generally, the release of CO2 from spring water like that seen above explains why so much calcium carbonate has precipitated to form all the travertine that we're seeing here along the stream.
Let's look at these travertines in more detail. The image below shows broken surfaces of two samples.
|
|
|

|
|
|
|
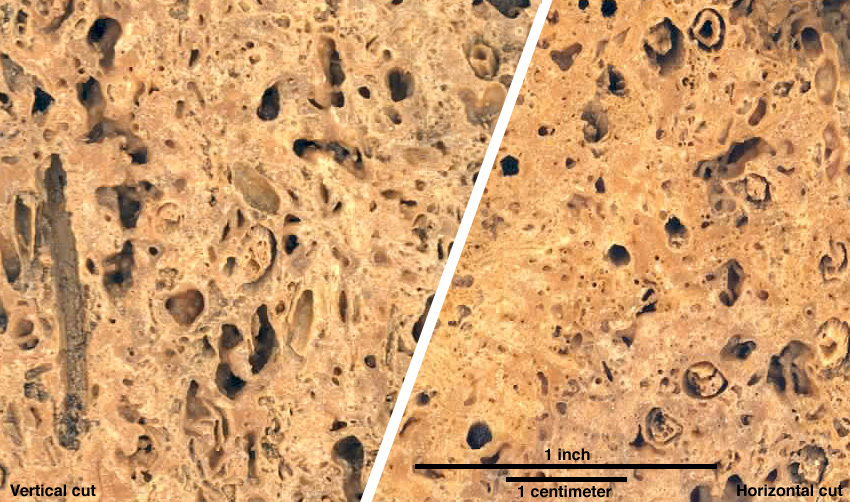
The image below shows two cuts through the sample above at the left. At left below is a vertical cut, and at right is a horizontal cut. What differences do you see, and do they make sense in terms of travertine development?
|
|
|
|
|
|
|
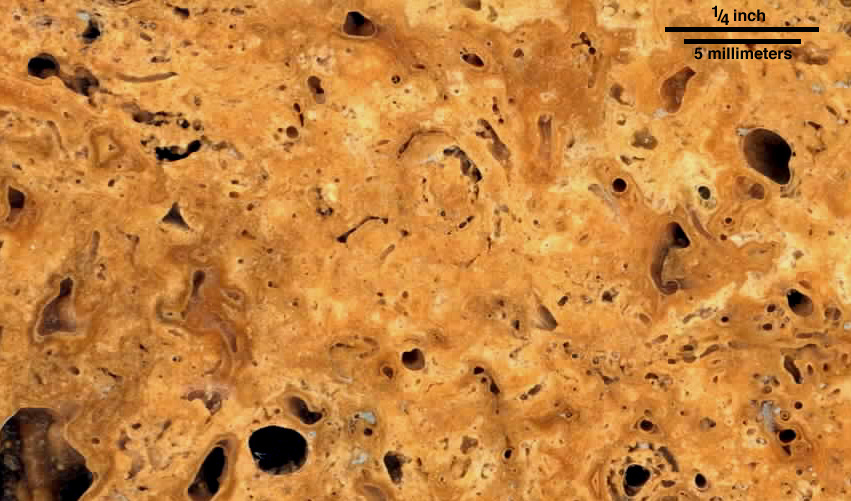
So what do you see? In the image on the left above, there is a striking vertical cylindrical mold, probably of a plant stem, at the far left. Other molds enter the plane of the image at low angles and so are nearly vertical - there's a nice example at upper right. On the other hand, in the image on the right, there are several circular features that are probably transverse cuts through these molds. Both images fit the idea of calcium carbonate coating near-vertical plant stems and precipitating in masses between those stems. With that in mind, we can look below at higher-resolution image of a horizontal cut. What do you see?
|
|
|
|
|
|
|
If you look closely at the image above, you'll see that there's a lumpy or peloidal texture within the calcium carbonate that makes of most of the travertine. That kind of clumpiness is commonly attributed to bacteria that may help induce precipitation of the carbonate, generating masses of bacterium-sized crystals within any one small aggregate. The two images below are of another piece of travertine from Falls Creek, the one on the right in the image of broken surfaces above. The second image below is a high-resolution view of a small area within the first.
|
|
|

|
|
|

|
|
|
|
The images are different, but the key observations remain mostly the same: this material is porous and consists of calcium carbonate that has coated plant stems and made small concretions. If you read our definitions of travertine and tufa above and if you've been looking at these images, you may have come up with this question: is this really travertine? The calcium carbonate is definitely porous, and we can clearly see evidence of plants around which the carbonate precipitated. That fits our definition of tufa, rather than travertine. In fact, workers at UGA familiar with such deposits have called these samples tufa. However, published papers and theses about the deposits along Fall Creek contain the word "travertine", and not "tufa", in their titles. Clearly one person's travertine can be another's tufa. With that thought, we'll end our visit to Fall Creek. You're encouraged to go on to the companion trip in this pair of field trips. That second trip is to a much larger, hotter, and more famous spring, the Mammoth Hot Springs of Yellowstone National Park. See you there!
|
|
|
Back to The main page for Railsback's virtual field trips
Back to Railsback's main page
Back to the UGA Geology Home Page