|
This is the second in a pair of virtual field trips to localities in the western United States at which travertine is forming today. This trip takes us to northwestern Wyoming, and specifically to Mammoth Hot Springs in Yellowstone National Park. The other trip is along Fall Creek in southeastern Idaho.
|

|
|
|
|
So what's travertine? Definitions vary, but a relatively restrictive modern definition might be "a body of calcium carbonate precipitated from waters of a hot spring, and thus relatively solid and not porous". Our trip takes us to a very hot spring, and big one too: it's Mammoth Hot Springs, a major attraction in the northernmost reaches of Yellowstone National Park. Here's a view of Mammoth Hot Springs from below. The trees should give a sense of the scale: this is truly "Mammoth":
|
|
|

|
|
|
|
Our next view, in the image below, is from higher up, and from the side of the deposit. Note the many white stair-steps of fresh calcium carbonate.
|
|
|

|
|
|
|
For the image below, we've moved a little farther down along the side of the travertine mass. Here you can see that there are pools of water dammed above many of the "stairsteps" that we saw earlier. Note the steam rising at the upper right to get a sense of the temperature of that water.
|
|
|

|
|
|
|
In our last view from the side, seen in the image below, we seem more rimmed pools. Note the stalactite-like spikes of calcium carbonate hanging from the rim of the large pool at the upper left.
|
|
|

|
|
|
|
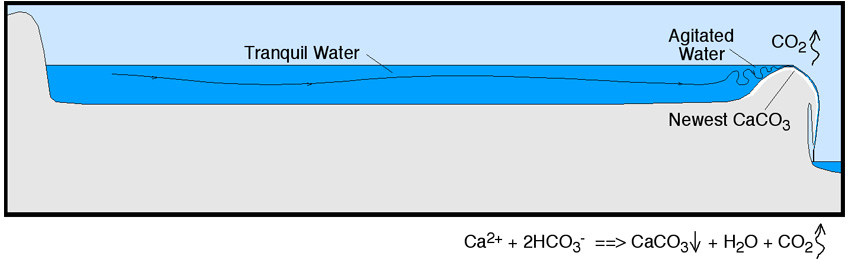
So what causes these rimmed pools, and these stalactite-like deposits around the rims? The answer again has everything to do with agitation of the water, release of CO2, and resultant precipitation of calcium carbonate. In the pool behind the rimstone dams, the water is tranquil, little CO2 exsolves, and so little calcium carbonate is deposited. However, as the water flows over the edge, it is stirred up and it releases CO2. The same chemical reaction goes into effect again: the removal of CO2 from the water pulls the reaction to the right, so that the water precipitates calcium carbonate as it goes ever the edge. That builds the dam and, with any precipitate left over, the spikes hanging down outside the dam.
|
|
|
|
|
|
| As a last illustration of the precipitation of calcium carbonate here, let's go down to the boardwalk at the base of Mammoth Hot Springs and look at the posts that support the boardwalk. Notice how the travertine has coated the timbers of the boardwalk, evidence of an active and rapid process. Note also the small rimstone dams to the left. |
|
|

|
|
|
|
So - on this trip and on our other travertine trip, we've visited two localities at which travertine is forming today. At Mammoth Hot Spring, the waters are so hot that little macroscopic life survives, and so a massive deposit with little porosity forms. On the other hand, at our first locality along Fall Creek, cooler water allows vegetation to flourish, and molds of that vegetation make a more porous travertine or tufa. At both locations, however, exsolution of CO2 is demonstrably responsible for driving precipitation of calcium carbonate to produce the deposits that we've seen.
|
|
|
|
|
Back to The main page for Railsback's virtual field trips
Back to Railsback's main page
Back to the UGA Geology Home Page